Publications
Teratology Primer, 3rd Edition
Can Prenatal Exposures Affect Brain Development and Behavior?
Charles V. Vorhees, Ph.D. and Michael T. Williams, Ph.D.
Division of Pediatric Neurology, Dept. of Pediatrics, Cincinnati Children’s Hospital Medical Center and University of Cincinnati, Cincinnati, OH
For decades embryonic and fetal injuries were conceived in terms of birth defects (congenital malformations). Effects on the central nervous system (CNS) were recognized if the brain was visibly affected or the child was severely intellectually disabled. This view began to change in the 1960-70s.
We know from our own experience that cognitive development is a long process; think how long you have spent in school. From functional neuroimaging studies we know that the prefrontal cortex, where planning and judgment are mediated, does not fully mature until approximately 24 years of age. This protracted development means that the brain is potentially vulnerable to having these developmental processes perturbed from the start of embryogenesis all the way to young adulthood.
The concept that chemicals can damage the developing brain (neurobehavioral teratogenesis or developmental neurotoxicity) is today an accepted fact. How did this recognition emerge? There were a series of environmental and drug effects that occurred that damaged the developing brain of many children, and these effects or “mistakes” created an awareness that prenatal and later developmental insults to the nervous system can have long lasting effects. Moreover, these functional effects can be observed even if the cellular basis for them is not known at the time the effects are detected. Finding the cellular and molecular basis of changes in how the brain functions is difficult, and because access to the brain is limited, we often lack the means to “see” into the brain in enough detail to find exactly where the problem is or how it originated. Brain imaging methods, particularly functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), are changing this ability to see, but these methods are still not at the level of precision needed to identify most brain disorders.
One of the first environmental problems that brought functional brain changes to people’s attention was identified because of mercury contamination in Minamata Bay in Japan. People living around the bay relied on fish as a staple of their diet. The mercury discharged by factories around the lake settled to the bottom where it was transformed to methylmercury in plants and concentrated progressively as it worked its way up the food chain to the largest fish, and these fish were consumed by those living near the lake. As the methylmercury accumulated in people around the bay, they began to show symptoms. People of all ages showed symptoms, but the most severe were seen in children, especially in newborns. The effects included cognitive impairment, cerebral palsy, blindness, coordination problems, and other deleterious effects. Methylmercury poisoning has since been reported in other places; each time the most severely affected were children who were prenatally exposed. Even women who ingested moderate amounts had affected children despite showing no symptoms themselves, demonstrating that the developing brain is more vulnerable than the mature brain.
The heavy metal lead is the best known chemical that causes CNS impairments in the US. Adverse effects are seen after prenatal or childhood exposure or a combination of both. Behavior was found to be the most sensitive end point for the effects of lead. In fact, an entirely safe level of lead remains to be determined; at present there is no safe level of exposure for children. Lead is illustrative of developmental neurotoxicity for several reasons: (1) it was a pervasive element for decades, and it remains a problem in urban areas today; think of Flint, Michigan, Washington D.C., and Los Angeles and the revelations that lead is in the water of many of cities and neighborhoods unbeknownst to residents, despite the fact that lead was removed from gasoline, paint, and other products decades ago. The effects of lead are still being felt because removing lead from old houses is costly and time-consuming; lead is in buried layers of paint, in dust and soil, and in water pipes and is difficult and expensive to remediate; (2) lead is a global problem affecting most industrialized countries; and (3) lead effects occur over a long period of development because lead is sequestered in the body and maturation of the brain is slow, creating a long exposure period.
Other environmental agents that cause developmental neurotoxicity with sufficient exposure include the heavy metal cadmium, arsenic, manganese, polychlorinated biphenyls, and some pesticides, and there are suspicions about some of phthalates in plastics, bisphenol A from incinerators, and polybrominated diphenyl ether flame retardants.
What about drugs? Drugs are designed to have biological effects so it is no surprise that of the all drugs sold, some unexpectedly turn out to cause birth defects including neurotoxic effects. The most infamous teratogen is thalidomide. Thalidomide was sold as a general sleep aid and pregnancy anti-nausea medication and was thought to be so safe that it was approved in Germany and several other countries to be sold over-the-counter. But when taken during the first trimester, thalidomide caused limb reduction defects and other birth defects. Later evidence showed it also was associated with increased prevalence of autism.
Two legal, recreational drugs can also adversely affect brain development and behavior: alcohol and tobacco. Both can result in life-long neurobehavioral problems. At the upper end of exposure, prenatal alcohol causes the Fetal Alcohol Syndrome (or Fetal Alcohol Spectrum Disorder; FASD), characterized by three clusters of effects: (a) growth impairment (pre- and postnatal), (b) facial dysmorphogenesis, and (c) brain/behavioral effects. The latter include changes in structure (using brain imaging) and behavior of affected individuals (intellectual impairment, attention-deficit hyperactivity disorder (ADHD), emotional instability, and antisocial behavior). There are also less severe effects, called fetal alcohol effects (FAE), in which one or two of the clusters are observed but not all three. Cigarette smoking is associated with intrauterine growth restriction and reductions in IQ, estimated to be about 10 points in verbal ability. Animal experiments recapitulate that prenatal nicotine affects offspring behavior and neurotransmitters.
What about illegal drugs such as cocaine, methamphetamine, LSD, heroin, PCP, and others? We know these drugs cross the blood brain barrier and the placenta so the embryo and fetus are exposed, but do they cause harm? Some of these drugs, e.g., methamphetamine, accumulate in the fetus more than in the mother. Despite this accumulation, effects on brain development have been difficult to prove. These drugs do not cause congenital malformations. However, animal studies show that prenatal cocaine alters dopamine systems, including its principal receptor, the dopamine D1 receptor. Changes in D1 receptor expression because of increased receptor internalization in the neuron makes the receptor less available to interact with dopamine when it is released from the synaptic terminal. Changes are also seen in the neurotransmitter GABA in pyramidal neurons in the cerebral cortex. It is believed that these effects in combination lead to the adverse behavioral outcomes.
In children prenatally exposed to cocaine, problems of attention, such as ADHD, are prominent; this deficit is also consistent with dopamine being affected since dopamine plays a significant role in attention, activity, and impulsivity. Children prenatally exposed to cocaine have IQ reductions but these are fairly small; approximately 4 points. Other studies find increased delinquent behavior, reduced problem solving, and impaired abstract reasoning. These effects are not easily noticed on an individual basis, but on a population basis they translate to more problems for affected children in school, reduced rates of college attendance and completion, and reduced success at getting and retaining jobs. If the bell-shaped distribution of these traits is shifted downward by prenatal drug exposure, it means thousands of children falling into categories needing special education that would not otherwise be needed; therefore, effects such as these can have large impacts at a societal level. Patterns of use are important for drug effects; those using higher doses or more drugs (polydrug abuse) for longer times during gestation and obtaining less prenatal care make outcomes from cocaine exposure worse; these effects are likely to be the case for other drugs of abuse as well.
Prenatal marijuana has also been documented to result in reductions in visual processing and impulse control. Given the legalization of marijuana in several jurisdictions in the US and elsewhere in recent years, combined with development of more varied and potent strains, we are engaged in a large-scale social experiment with unknown consequences for children exposed during early development. A recent review of what is known about the prenatal effects of marijuana indicates that there are long-term effects.
Methamphetamine abuse became a problem about a decade after the upsurge in cocaine/crack use. Methamphetamine causes changes in brain structure, neurotransmitters, spatial memory, and language development after prenatal exposure, and the problem is significant. Between 1994 and 2006 pregnant women entering drug treatment programs in the US. who identified methamphetamine as their primary drug of abuse rose from 8% in 1994 to 24% in 2006. Whether this trend continued after 2006 is not clear as there are no new data. Moreover, the only human prospective prenatal study on methamphetamine has been in progress for about 10 years, and the children of these pregnancies are now about 7.5 years told. So far it has been reported that the exposed children show more externalizing and aggressive behavior and several cognitive problems. Animal experiments reveal long-term effects on brain neurochemistry and behavior, including impaired spatial memory and egocentric learning and enduring changes in dopamine and serotonin after developmental exposure.
What about prescription drugs? Isotretinoin (13-cis-retinoic acid) is prescribed for the treatment of severe acne. Within a few years of its becoming available, cases of children with birth defects that were ultimately identified as retinoid embryopathy appeared. In addition to birth defects, many of these children had low IQs once they were old enough to be tested. In animal experiments, Vitamin A (another retinoid) is known to cause neurobehavioral impairments. While isotretinoin was predicted to be a teratogen and carried strong warnings against use in pregnancy, people obtained the drug from friends or had unplanned pregnancies and did not understand the risks. Hence, the impairments could have been predicted based on preclinical data in rats but were not adequately appreciated.
The effects of isotretinoin on intelligence and birth defects are not aligned, i.e., there are children with severe birth defects and mental impairments, but others that have minor birth defects but severe intellectual impairments. This mismatch between malformations and brain effects is important, because for many years it was believed that testing for birth defects was sufficient to ensure drug safety. The idea was that if a drug caused birth defects it would or could cause behavioral effects; the reverse idea, that the absence of birth defects indicated that the drug would not cause behavioral effects, was incorrect. Isotretinoin dispelled this belief and demonstrated that it takes longer to identify neurobehavioral effects than it does to find birth defects since birth defects are seen at or within a year after birth but it takes years to prove a connection between exposure to a drug and behavioral defects. When a drug does not cause a birth defect, proving a connection later when children are 7, 10, or 12-years-old and relating the problem back to prenatal exposure is difficult. The fact is that measuring learning, memory, attention, and other cognitive abilities cannot be accurately done until children are older, by which time parents’ memories are not very accurate, and medical records are not always complete.
Isotretinoin is also an example of a drug that has therapeutic value for the intended use but detrimental consequences for an unintended recipient, i.e., the embryo and fetus. The disconnect between the effects on the adult and the embryo and fetus lies at the heart of why this area of safety has proven to be difficult. There remains debate between those who want more safety testing and those who want less; the latter argue that CNS deficits such as those caused by isotretinoin are rare, the tests are expensive, less than perfect, time-consuming, add to the high cost of drug development, and delay helpful drugs from reaching those who need them. On the other side is the argument that while drug-induced developmental neurotoxicity may not be common, when it occurs the effects are detrimental and irreversible to those who are affected.
How big is the problem? The effects of isotretinoin are striking but it is used by very few so the societal impact is not large. A larger problem occurs in epilepsy. Approximately 1% of people (~3 million) have epilepsy and most are treated with antiepileptic drugs (AED). Prenatal AED exposure is associated with several birth syndromes. The clearest example is valproate (Depakote), an AED that when taken during pregnancy leads to 1-2% of exposed infants born with neural tube defects and a higher percentage born with other malformations. The syndrome includes dysmorphic faces and impaired intellectual development; valproate also may increase rates of autism spectrum disorder (ASD).
AED use is widespread, but even so these drugs are also used by a minority of people. What about common drugs that are taken by millions? While it is reassuring that no evidence of fetal neurotoxicity has yet appeared for most drugs, determination of safety for brain development during pregnancy is often absent. It takes decades to find behavioral and cognitive problems after prenatal drug exposure when the effects are severe and longer if the effects are subtle. Associating problems with learning, attention, externalizing behaviors, and executive function takes decades to establish. The good news is that animal and epidemiological study methods are improving, making the detection of neurobehavioral effects better.
For exposures that are teratogenic, the first trimester is a period of high vulnerability, often before pregnancy is recognized. This period of development is called embryogenesis or organogenesis. For the CNS, the period of vulnerability starts during the first trimester and lasts throughout pregnancy, childhood, and to adolescence. The reason for this vulnerability is the complexity and duration of brain and behavioral development (Figure 1).
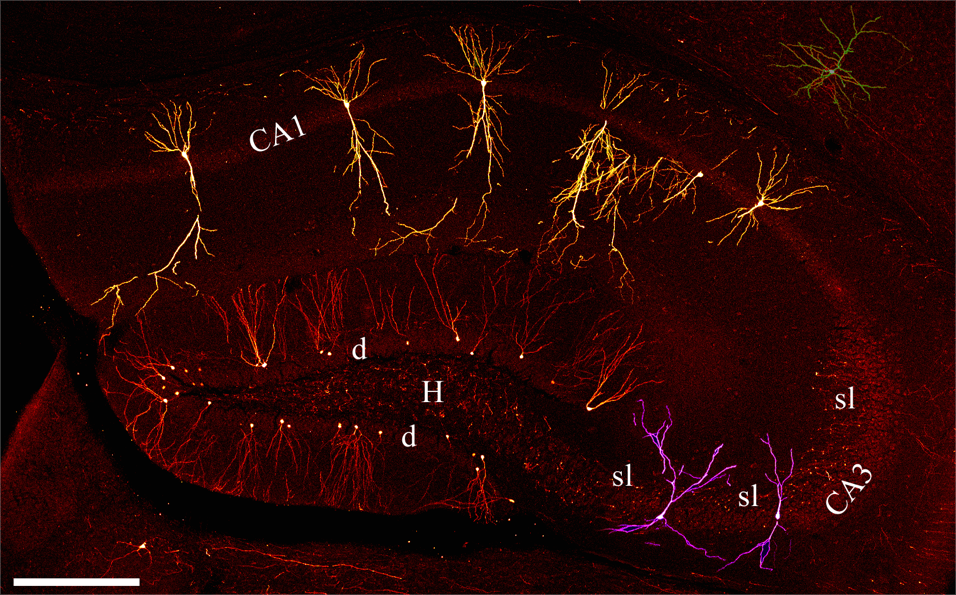
Figure 1. From review by S. Danzer with permission {Danzer, 2008 #2404}. Photomontage of confocal microscopy images showing principal neurons of the hippocampal. Images are from an adult Thy1-GFP-expressing mouse. Red = dentate granule cells, Purple = CA3 pyramidal cells, Yellow = CA1 pyramidal cells. d = dentate granule cell layer; H = hilus; sl = stratum lucidum; CA3 = CA3 pyramidal cell layer; CA1 = CA1 pyramidal cell layer. Scale bar = 300 μm.
Developmental neurotoxicity can sometimes be subtle but it is worth remembering that what is subtle to an observer may not be subtle to those who are affected. ADHD is often regarded as subtle compared to a life-threatening cardiac defect. But many heart defects can be surgically repaired without long-term consequences. But how can ADHD, ASD, learning impairment, or intellectual disability be repaired? The risks and benefits of drugs, commercial chemicals, pesticides, or factory effluents can be difficult to weigh with precision but the precautionary principle should prevail by erring on the side of caution. To that end, developmental neurotoxicity assessments are important as part of the safety net. This field is in need of more research, not only to find better ways to detect such effects but to understand the mechanisms that underlie how certain chemicals disrupt brain development and behavior.
Suggested Reading
Adams J, Lammer EJ (1993) Neurobehavioral teratology of isotretinoin. Reprod Toxicol 7:175-177.
Ardinger HH, Atkin JF, Blackston RD, Elsas LJ, Clarren SK, Livingstone S, Flannery DB, Pellock JM, Harrod MJ, Lammer EJ, . (1988) Verification of the fetal valproate syndrome phenotype. Am J Med Genet 29:171-185.
Bhang SY, Cho SC, Kim JW, Hong YC, Shin MS, Yoo HJ, Cho IH, Kim Y, Kim BN (2013) Relationship between blood manganese levels and children's attention, cognition, behavior, and academic performance--a nationwide cross-sectional study. Environ Res 126:9-16.
Chang LW (1994) Principles of Neurotoxicology. New York: Marcel Dekker, Inc.
Danzer SC (2008) Postnatal and adult neurogenesis in the development of human disease. Neuroscientist 14:446-458.
Diaz SD, Smith LM, LaGasse LL, Derauf C, Newman E, Shah R, Arria A, Huestis MA, Della Grotta S, Dansereau LM, Neal C, Lester BM (2014) Effects of prenatal methamphetamine exposure on behavioral and cognitive findings at 7.5 years of age. J Pediatr 164:1333-1338.
Eze N, Smith LM, LaGasse LL, Derauf C, Newman E, Arria A, Huestis MA, Della Grotta SA, Dansereau LM, Neal C, Lester BM (2016) School-Aged Outcomes following Prenatal Methamphetamine Exposure: 7.5-Year Follow-Up from the Infant Development, Environment, and Lifestyle Study. J Pediatr 170:34-38 e31.
Frederick AL, Stanwood GD (2009) Drugs, biogenic amine targets and the developing brain. Dev Neurosci 31:7-22.
Fried PA, Watkinson B, Gray R (2003) Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 25:427-436.
Jablonski SA, Williams MT, Vorhees CV (2015) Neurobehavioral Effects from Developmental Methamphetamine Exposure. Curr Top Behav Neurosci.
Jablonski SA, Williams MT, Vorhees CV (2016) Mechanisms involved in the neurotoxic and cognitive effects of developmental methamphetamine exposure. Birth Defects Res C Embryo Today 108:131-141.
Lam J, Lanphear BP, Bellinger D, Axelrad DA, McPartland J, Sutton P, Davidson L, Daniels N, Sen S, Woodruff TJ (2017) Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-analysis. Environ Health Perspect 125:086001.
Lammer EJ, Sever LE, Oakley GP, Jr. (1987) Teratogen update: valproic acid. Teratology 35:465-473.
Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, Curry CJ, Fernhoff PM, Grix AW, Jr., Lott IT, et al. (1985) Retinoic acid embryopathy. N Engl J Med 313:837-841.
Lanphear BP, Dietrich K, Auinger P, Cox C (2000) Cognitive deficits associated with blood lead concentrations <10 microg/dL in US children and adolescents. Public Health Rep 115:521-529.
Oulhote Y, Bouchard MF (2013) Urinary metabolites of organophosphate and pyrethroid pesticides and behavioral problems in Canadian children. Environ Health Perspect 121:1378-1384.
Richardson GA, Goldschmidt L, Larkby C, Day NL (2015) Effects of prenatal cocaine exposure on adolescent development. Neurotoxicol Teratol 49:41-48.
Riley EP, Vorhees CV (1986) Handbook of Behavioral Teratology. New York: Plenum.
Slikker W, Chang LW (1998) Handbook of Developmental Neurotoxicology. San Diego: Academic Press.
Sobrian SK (2016) Developmental cannabinoid exposure: New perspectives on outcomes and mechanisms. Neurotoxicol Teratol 58:1-4.
Terplan M, Smith EJ, Kozloski MJ, Pollack HA (2009) Methamphetamine use among pregnant women. Obstet Gynecol 113:1285-1291.
Thompson BL, Levitt P, Stanwood GD (2009) Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci 10:303-312.
Vorhees CV (1986) Retinoic acid embryopathy. New Engl J Med 315:262-263.
Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van GA, Slavkovich V, Loiacono NJ, Cheng Z, Hussain I, Momotaj H, Graziano JH (2004) Water arsenic exposure and children's intellectual function in Araihazar, Bangladesh. Environ Health Perspect 112:1329-1333.
Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Ahsan H, Levy D, Kline J, van Geen A, Mey J, Slavkovich V, Siddique AB, Islam T, Graziano JH (2011) Arsenic and manganese exposure and children's intellectual function. Neurotoxicology 32:450-457.
